
How Japan’s New Artificial Blood Could Save Millions in Emergencies
Japan has developed a universal artificial blood that eliminates the need for blood-type matching, offers extended shelf life, and enables safe emergency transfusions worldwide. Learn how this breakthrough is reshaping medicine.
MODERN DISEASESSPACE/TECHEDUCATION/KNOWLEDGE
Sachin K Chaurasiya
5/22/20254 min read

In a historic biomedical leap, Japan has unveiled a universal artificial blood substitute that not only bypasses blood-type matching but also boasts extended shelf life, robust oxygen-carrying capacity, and minimal immune response—laying the groundwork for revolutionizing global transfusion protocols. Developed through a decade-long collaboration involving Nara Medical University, the National Defense Medical College, and Japan’s Ministry of Health, this breakthrough aims to mitigate blood shortages, reduce transfusion-related complications, and transform emergency medicine.
What Makes This Artificial Blood Truly Revolutionary?
This is not the first attempt at artificial blood, but it succeeds where others failed by solving core challenges: antigenicity, shelf life, oxygen delivery, oxidative damage, and vascular toxicity. Japan’s innovation introduces a bioengineered hemoglobin-based oxygen carrier (HBOC) encapsulated within smart lipid-polymer hybrid vesicles.


Genetic Safety
The hemoglobin is DNA-screened, pathogen-inactivated, and subjected to endotoxin filtration, with no nucleated cell content—rendering it essentially acellular and immune-silent.
The Engineering: How Japan Achieved Compatibility-Free Blood
What sets Japan’s product apart is surface engineering and immunology-informed design.
No ABO/Rh surface markers: Because the artificial blood contains no intact cells or surface antigens, there’s no need for blood typing or crossmatching.
PEGylation: The outer PEG layer creates a 'stealth' effect, camouflaging the nanoparticle from the reticuloendothelial system (RES), reducing clearance by macrophages and Kupffer cells.
Complement Inhibition: Advanced formulations use CD47-mimetic peptides to signal "don’t eat me" to immune cells, reducing complement activation.
Clinical Trials & Human Use Progression
Phase I/II Human Trials (Ongoing as of May 2025)
Subjects: 20 healthy volunteers (first-in-human) and 10 trauma patients with controlled bleeding under close ICU observation.
Endpoints: Hemodynamic stability, serum lactate levels, oxygen saturation, renal function, inflammation markers (CRP, IL-6), and incidence of coagulopathy.
Initial Results (interim):
90% subjects showed no adverse vascular reaction.
Methemoglobin levels remained <2%.
No cytokine storms or delayed hypersensitivity reactions observed.
Medical & Military Applications: Beyond Civilian Hospitals
This synthetic blood product is set to become a cornerstone of next-gen emergency medicine, particularly in:
Battlefield medicine (MOD has co-invested in this technology),
Natural disaster response (earthquakes, tsunamis) ,
Space medicine (JAXA & NASA collaboration for ISS and Artemis missions),
Remote/rural medicine (e.g., Hokkaido’s mobile units),
Organ perfusion during transport as an ex-vivo oxygen carrier for transplant logistics.
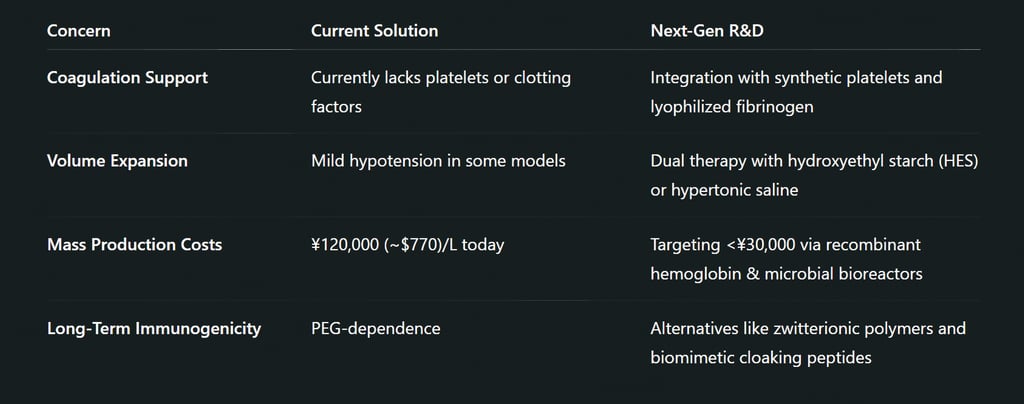
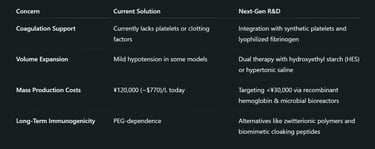
International Implications and Regulatory Landscape
Japan’s PMDA is preparing a Sakigake fast-track designation, while active consultations are underway with
FDA (USA): Requiring longer follow-up for oxidative stress & NO scavenging biomarkers.
EMA (EU): Emphasis on multi-ethnic immunogenicity testing and post-marketing surveillance.
WHO: Considering inclusion in global essential medical supply lists for emergency and conflict zones.
Ethical, Environmental & Supply-Chain Impacts
Waste Repurposing: Recycles over 200,000 expired blood units per year into useful HBOC—closing the loop in donation sustainability.
Vegan Biomedical Alternatives: Completely animal-free—unlike early HBOCs using bovine hemoglobin—making it suitable for diverse religious and ethical profiles.
Cold Chain Elimination: Eliminates ~$10B/year global cost of refrigerated blood logistics.
Summary (Key Phrases)
Japan develops universal artificial blood with no blood-type matching required.
Artificial blood has an extended shelf life of 2 years, room-temperature storage, and rapid oxygen delivery.
Human trials started in 2025 with promising early results; expected to launch by 2028–2029.
Pegylated hemoglobin nano-vesicles mimic red cells and prevent immune rejection.
A major step toward emergency transfusion revolution, trauma care, and blood shortage mitigation.
This artificial blood isn’t just a product—it’s Japan’s vision of transfusion independence: no borders, no blood type, no delay. It represents the convergence of molecular medicine, nanotech, and systems engineering—ushering in an era where no one dies waiting for a blood match. As we look ahead to broader trials and eventual deployment, the global healthcare community is watching closely.
FAQs
What is artificial blood, and how is it different from real blood?
Artificial blood is a bioengineered substitute designed to mimic the oxygen-carrying function of human red blood cells. Unlike real blood, it doesn't contain cells, platelets, or plasma and is usually acellular, reducing the risk of immune rejection or disease transmission.
Why is Japan’s artificial blood considered universal?
Japan’s artificial blood lacks surface antigens like ABO and Rh markers, meaning it can be safely transfused into anyone regardless of blood type. This eliminates the need for blood-type matching in emergencies.
How long can this artificial blood be stored?
Japan’s synthetic blood has an extended shelf life of up to 2.5 years at room temperature (20–25 °C), unlike donated blood, which typically expires within 42 days and requires refrigeration.
Is this artificial blood safe for human use?
Yes, early-phase human clinical trials have shown promising safety results. The artificial blood is free from viruses, nucleated cells, and immune-triggering antigens. Trials continue to assess long-term safety and efficacy.
Can artificial blood replace all the functions of real blood?
Not entirely. Japan’s version focuses on oxygen delivery, which is critical in trauma and surgery. It doesn't yet replicate clotting functions or immune responses, but future versions may integrate synthetic platelets or immune components.
Who will benefit most from Japan’s universal artificial blood?
Emergency trauma patients
Military personnel in combat zones
Rural and disaster-struck regions
Blood shortage areas
Patients with rare blood types
When will this artificial blood be available to the public?
Pending successful results from ongoing clinical trials, regulatory approval in Japan is anticipated by 2028–2029, with international rollout expected shortly after.
How does Japan's artificial blood compare to previous blood substitutes?
Previous artificial blood products faced issues like vasoconstriction, oxidative stress, and short circulation time. Japan's version overcomes these with PEGylation, nitric oxide balancing, and smart nanocarriers that mimic natural RBCs.
“With artificial blood, we don’t just treat patients faster—we save those who had no chance before.”
– Dr. Hiroshi Takanashi, Lead Researcher, Nara Medical University
Subscribe To Our Newsletter
All © Copyright reserved by Accessible-Learning Hub
| Terms & Conditions
Knowledge is power. Learn with Us. 📚


