
Babies Born after Mitochondrial Donation Treatment: A Scientific Breakthrough with a Human Touch
Discover how mitochondrial donation treatment is giving hope to families with genetic disorders. Learn about the science, ethics, and future of babies born using three-parent IVF technology.
HEALTH/DISEASEEDUCATION/KNOWLEDGENEWS/CURRENT AFFAIRS
Kim Shin
7/20/20255 min read
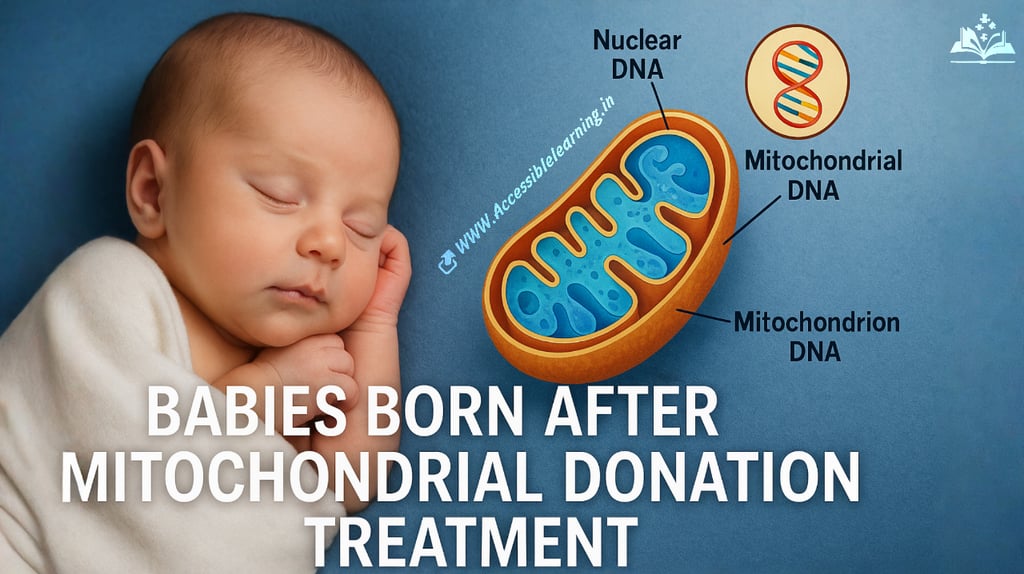
In recent years, a revolutionary technique in reproductive science has caught global attention—mitochondrial donation treatment (MDT), also dubbed by many as "three-parent baby technology." This procedure is not just about pushing the boundaries of science; it’s about saving lives, preventing the transmission of deadly mitochondrial diseases, and offering hope to families burdened by inherited disorders.
As the first few babies born through this method take their first breaths, the world watches with awe, caution, and curiosity.
What is Mitochondrial Donation Treatment?
Mitochondria are tiny structures within cells that produce energy—think of them as cellular power plants. Some women carry mutations in their mitochondrial DNA (mtDNA), which can lead to severe, often fatal, conditions like:
Leigh syndrome
MELAS (mitochondrial encephalopathy)
Myoclonic epilepsy
Mitochondrial myopathy
These diseases affect vital organs, especially the brain and muscles, and often appear in infancy or early childhood.
Mitochondrial Donation Treatment (MDT) is a technique that allows women with faulty mitochondria to have genetically related children without passing on mitochondrial disorders. It involves replacing faulty mitochondria with healthy ones from a donor egg.
How the “Three-Parent Baby” Technique Works
The term “three-parent baby” refers to the fact that a child conceived through MDT carries DNA from three individuals:
Nuclear DNA (from mother)—99.9% of genetic material
Nuclear DNA (from father)—50% of genetic material
Mitochondrial DNA (from donor woman)—only about 0.1% of total DNA
Two Primary Techniques Used:
Maternal Spindle Transfer (MST)—Done before fertilization
The nucleus is removed from the mother's egg and placed into a donor egg (with healthy mitochondria) whose nucleus has been removed. Then fertilized with the father’s sperm.
Pronuclear Transfer (PNT)—Done after fertilization
Both the mother’s and donor’s eggs are fertilized. The nucleus of the fertilized mother’s egg is transferred into the donor’s fertilized egg (which has had its nucleus removed).
These processes are performed under strict laboratory conditions and involve high precision to avoid DNA mixing errors.
Milestone: The First Babies Born Using MDT
The first successful births using MDT took place in Newcastle, UK, and Mexico, with other cases emerging globally in tightly regulated medical trials.
UK (2023): The UK’s Human Fertilisation and Embryology Authority (HFEA) confirmed that a handful of babies had been born via MDT in licensed clinical settings under strict guidelines.
Mexico (2016): A team led by Dr. John Zhang achieved the world’s first birth using maternal spindle transfer, though it raised ethical concerns due to regulatory gaps.
These babies were born healthy, showing no signs of mitochondrial disease—a significant step forward for reproductive genetics.
The Ethical & Social Debate
While the science is promising, mitochondrial donation raises important ethical questions:
Is this Genetic Engineering?
No. The technique doesn’t alter nuclear DNA (which determines physical traits, intelligence, etc.)—it only replaces faulty mitochondria. But critics worry it opens the door to designer babies.
Consent & Identity
The child technically has DNA from three people. Should they be informed? What rights does the mitochondrial donor have?
Long-Term Effects
Since the technology is new, long-term data on children born through MDT is limited. Could subtle cellular interactions cause issues later in life?
Accessibility & Cost
MDT is expensive and not widely available. There’s growing concern over health equity—only the wealthy might access this cutting-edge therapy.
Legal & Global Regulatory Status
United Kingdom: Legal under the HFEA since 2015. The UK is the first country to formally license MDT.
United States: Not approved. The FDA prohibits clinical use of MRT (mitochondrial replacement therapy) without further review.
Australia and Canada: Currently under debate or restricted.
Mexico: No specific legal barriers, which is why early trials happened there.
Global opinions vary greatly, with countries either embracing, cautiously evaluating, or banning the procedure altogether.

The Future of Mitochondrial Donation
Mitochondrial donation is not just a medical marvel—it’s a lifeline for families affected by devastating diseases. Researchers continue to monitor the health of MDT-born children, develop better precision tools, and refine ethical frameworks.
Future developments may include:
Improved safety through AI-driven embryo analysis
Broader access in more countries
Public awareness campaigns to demystify the science
Clear donor-child relationship laws
The birth of babies through mitochondrial donation is a monumental step for medical science, but it’s also deeply human. It’s about parents who’ve lost children to cruel diseases getting another chance at life. It’s about ensuring that hope, not suffering, is inherited.
As science progresses, careful regulation, transparent dialogue, and empathy must guide us forward—so that innovation remains grounded in compassion.
FAQs
Q. What is a “three-parent baby”?
A "three-parent baby" is a child conceived using mitochondrial donation treatment (MDT), where DNA from three individuals is used:
The mother (nuclear DNA)
The father (nuclear DNA)
A female donor (mitochondrial DNA)
The donor's contribution is limited to about 0.1% of the child’s DNA, responsible only for energy production—not traits or personality.
Q. Why is mitochondrial donation needed?
This treatment is used to prevent the transmission of mitochondrial diseases, which are inherited from the mother and can cause severe or fatal conditions affecting the brain, muscles, heart, and organs. It allows affected women to have healthy, genetically related children.
Q. Is mitochondrial donation treatment legal everywhere?
No. It is only legal in a few countries, such as the UK, where it is highly regulated. In other places, like the U.S., it’s still under review or considered experimental. Some clinics in countries with loose regulations offer it privately.
Q. Are babies born through MDT genetically modified?
No. The procedure does not involve gene editing or modification. It simply replaces damaged mitochondria with healthy ones. The child’s traits and identity still come from the mother and father’s nuclear DNA.
Q. Is the donor considered a third parent?
Legally and genetically, no. The donor only contributes mitochondrial DNA, which doesn’t affect the child’s appearance, intelligence, or personality. Most jurisdictions do not grant parental rights or responsibilities to the donor.
Q. Are there any risks to the baby?
While early data is promising and most babies born via MDT are healthy, the long-term effects are still being studied. Risks include:
Potential mismatch between nuclear and mitochondrial DNA
Technical errors during embryo transfer
Unknown health outcomes later in life
Q. Can this treatment help with infertility?
Not directly. MDT is specifically for women who carry mitochondrial DNA mutations. However, research is ongoing into how similar techniques might support older women or those with poor egg quality in the future.
Q. How many babies have been born through mitochondrial donation so far?
As of 2025, fewer than 100 babies have been reported worldwide through this procedure, with most in the UK and experimental trials in Mexico, Greece, and Ukraine. Numbers remain low due to regulatory and ethical constraints.
Q. Is mitochondrial DNA passed to future generations?
Yes, if the baby is a girl, her healthy mitochondria will be passed on to her offspring. This means the benefits of MDT can be multigenerational, breaking the cycle of inherited mitochondrial disease.
Q. How expensive is mitochondrial donation treatment?
The cost can range from $20,000 to $50,000 or more, depending on the clinic, country, and procedures involved. It's generally not covered by insurance and may include travel, fertility treatment, and donor expenses.
Subscribe To Our Newsletter
All © Copyright reserved by Accessible-Learning Hub
| Terms & Conditions
Knowledge is power. Learn with Us. 📚


