
AlphaFold vs RoseTTAFold: The Battle of AI in Protein Structure Prediction!
Discover a fascinating comparison between AlphaFold and RoseTTFold, two cutting-edge AI tools transforming protein structure prediction. Learn their methodology, key differences, applications in science, and how they are revolutionizing biology and drug discovery.
HEALTH/DISEASEAI/FUTUREMODERN DISEASESA LEARNING
Sachin K Chaurasiya
10/29/20245 min read
In recent years, breakthroughs in artificial intelligence have revolutionized the field of biology, particularly in predicting protein structures. Two key tools—AlphaFold and RoseTTAFold—have gained worldwide recognition for their ability to predict 3D protein structures with unprecedented accuracy. Both tools are changing the way scientists approach biological research, drug discovery, and understanding of disease.
In this article, we will dive deep into AlphaFold and RoseTTAFold, find out how they work, what is the difference between them, and why they are so important to science. Let’s break it down in a way that is easy to understand and make sure it includes all the necessary details.

What Are AlphaFold and RoseTTAFold?
Both AlphaFold and RoseTTAFold are AI-powered tools designed to predict protein structures from their amino acid sequences. It is important to predict how a protein folds into its unique 3D shape because the structure determines its function. Historically, solving protein structures was a labor-intensive process that could take years. These tools change this by leveraging machine learning to predict structures quickly and accurately.
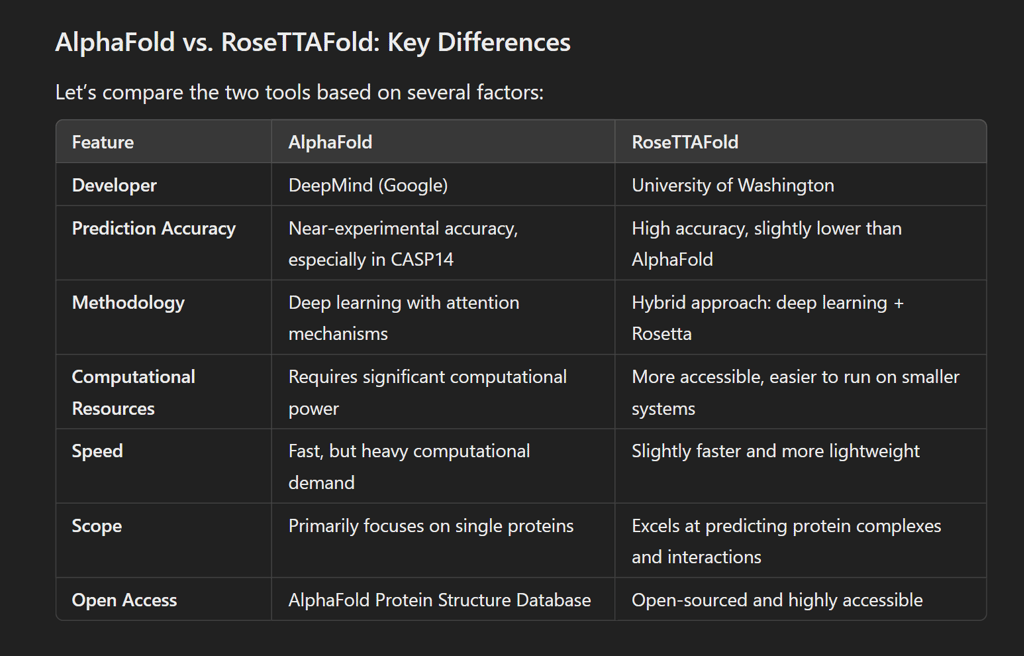
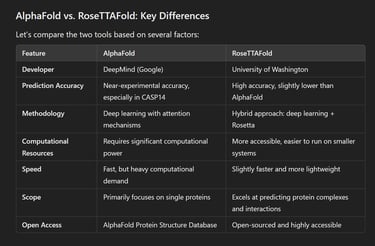
AlphaFold: Developed by Google DeepMind, AlphaFold uses deep learning to solve the “protein folding problem.”. AlphaFold 2, its improved version, sets a new benchmark in protein structure prediction by achieving almost experimental accuracy in many cases. It focuses on predicting the 3D coordinates of atoms in protein structures with extreme accuracy.
RoseTTAFold: Developed by the University of Washington, RoseTTAFold is an AI tool designed to predict protein structures using a hybrid approach. It combines machine learning with Rosetta—an established protein modeling software. RoseTTAFold also predicts interactions between proteins and can be used to design new proteins with specific functions.

How Do AlphaFold and RoseTTAFold Work?
While both tools aim for the same goal—predicting protein structures—their approaches differ significantly.
AlphaFold’s Approach
AlphaFold uses a deep learning architecture that includes an attention mechanism. The attention mechanism helps the model focus on important parts of the input sequence to predict accurate structural relationships. AlphaFold predicts the distances between pairs of amino acids and their relative angles, which allows it to create accurate 3D models.
Data Training: AlphaFold was trained on a vast database of protein sequences and known structures, which allows it to generalize and predict unseen protein structures.
Prediction Accuracy: AlphaFold produces highly accurate predictions, comparable to traditional experimental methods like X-ray crystallography or cryo-electron microscopy. It was a clear winner in the CASP14 (Critical Assessment of Structure Prediction) competition, achieving the highest accuracy.
RoseTTAFold’s Approach
RoseTTAFold integrates three tracks—sequence, distance, and 3D coordinates—together. This three-track system allows RoseTTAFold to optimize predictions more interactively. It uses deep learning models to predict how different parts of a protein are connected and how they fold together in space.
Hybrid models: Unlike AlphaFold, RosettaFold combines deep learning with traditional Rosetta modeling. Rosetta has been widely used in computational biology for years, making RosettaFold a unique blend of old and new methods.
Multi-tasking: RosettaFold not only predicts individual protein structures but can also model protein complexes and interactions, making it a versatile tool for studying large biological systems.
Accuracy & Performance!
The biggest question about AlphaFold and RoseTTAFold is accuracy. Both tools have performed well, but AlphaFold is ahead of the curve in terms of achieving experimental accuracy.
Accuracy of AlphaFold: AlphaFold 2 stunned the scientific community when it outperformed all other models during CASP 14. It achieved an average Global Distance Test (GDT) score of 92.4 out of 100, meaning its predictions were often as good as predictions obtained through laboratory experiments. This is a game-changer for fields such as drug discovery and molecular biology.
Accuracy of RoseTTAFold: RoseTTAFold is slightly less accurate than AlphaFold, but it remains competitive, especially when it comes to predicting large protein complexes or protein-protein interactions. Its three-track approach allows it to handle more diverse protein structures than AlphaFold, making it useful in specific research scenarios.
Applications in Science and Medicine
The impact of these tools is far-reaching, touching multiple fields:
AlphaFold Applications
Drug discovery: By predicting how proteins fold, AlphaFold helps scientists understand how drugs might interact with disease-related proteins. This speeds up the process of finding new treatments.
Disease research: Understanding protein structures can provide insight into diseases caused by misfolded proteins, such as Alzheimer's, Parkinson's, and some cancers.
Structural biology: AlphaFold has solved the structures of many proteins that were previously insoluble, accelerating research across a variety of biological disciplines.
RoseTTAFold Applications
Protein design: RoseTTAFold's strength lies not only in its ability to predict structure but also in its ability to design new proteins. This could lead to innovative solutions in biotechnology, such as designing proteins for industrial or therapeutic use.
Understanding complexes: RoseTTAFold is particularly useful for studying protein-protein interactions, which play important roles in cellular function and pathways.
Collaborative research: RoseTTAFold is open-source, making it accessible to more researchers around the world. This encourages collaboration and innovation across academic institutions.


Limitations & Challenges!
While both AlphaFold and RoseTTAFold have made incredible progress, they still face challenges.
Limitations of AlphaFold: Despite its impressive accuracy, AlphaFold requires a considerable amount of computational power, which can make it difficult for smaller labs or individual researchers to use independently. It also focuses primarily on individual proteins, meaning it may not always perform as well when predicting complex combinations or interactions.
Limitations of RoseTTFold: RoseTTAFold, while more accessible and versatile, does not match AlphaFold's accuracy in every scenario. It is best suited for academic research and scenarios where protein interactions are the focus, but for individual protein structures, AlphaFold is usually the better choice.
Future Prospects
The future of protein structure prediction looks promising with tools like AlphaFold and RoseTTAFold. Scientists are already exploring ways to combine their strengths. For example, some are using AlphaFold's high accuracy in single-protein predictions and RoseTTAFold's versatility in studying interactions, which opens the door to even more accurate and useful models.
Additionally, advances in AI and machine learning will continue to improve the speed and accuracy of these tools, making them more accessible to researchers and industries.


AlphaFold and RoseTTAFold represent a significant leap forward in computational biology. While AlphaFold is praised for its accuracy and has set a new gold standard for predicting individual protein structures, RoseTTAFold stands out for its flexibility and ease of use, making it a valuable tool in academic research and protein design.
Both tools are reshaping the landscape of science, giving researchers the ability to unlock the mysteries of life at the molecular level. Whether it's drug discovery, disease research, or protein engineering, AlphaFold and RoseTTAFold are pushing the boundaries of what is possible in modern biology.
Subscribe to our newsletter
All © Copyright reserved by Accessible-Learning
| Terms & Conditions
Knowledge is power. Learn with Us. 📚


