
78th World Health Assembly of WHO Formally Adopts the World’s First Pandemic Agreement
The 78th World Health Assembly adopts WHO’s first-ever Pandemic Agreement, aiming to enhance global preparedness, equity, data sharing, and rapid response.
MODERN DISEASESHEALTH/DISEASEGLOBAL ISSUESNEPOTISM/SOCIAL ISSUES
Sachin K Chaurasiya
5/23/20256 min read


In a landmark decision, the 78th World Health Assembly (WHA) of the World Health Organization (WHO) formally adopted the world’s first Pandemic Agreement—a legally binding framework designed to strengthen international cooperation and readiness for future health crises. Held in Geneva, Switzerland in May 2025, the assembly marked a critical moment in the global health governance landscape following the hard lessons learned from the COVID-19 pandemic.
This groundbreaking move reflects a collective international will to ensure that the world never again faces a pandemic unprepared, uncoordinated, or unequally protected.
What is the Pandemic Agreement?
The Pandemic Agreement, also known as the WHO Pandemic Accord, is a comprehensive legal instrument that outlines how countries will:
Share critical information and resources during health emergencies
Coordinate responses under a common, transparent framework
Guarantee equitable access to vaccines, diagnostics, and treatments
Strengthen national and international preparedness systems
The agreement was crafted over two years of intense negotiations, debates, and consultations among WHO member states, global health experts, and civil society stakeholders.
Why Was This Agreement Needed?
The COVID-19 pandemic exposed severe weaknesses in global public health infrastructure:
Lack of coordination: Countries acted individually, often contradicting one another.
Inequitable access: Wealthier nations secured vaccines faster, leaving poorer nations behind.
Information gaps: Real-time data sharing was inconsistent, slowing down collective action.
These failures highlighted the urgent need for a common framework to manage future pandemics with more unity, speed, and fairness. The WHO Pandemic Agreement aims to close these gaps and lay the foundation for a more resilient global health system.
Key Highlights of the WHO Pandemic Agreement
Equity at the Core
One of the strongest pillars of the agreement is equity. The accord mandates mechanisms to ensure that developing countries are not left behind during global health crises. It sets up a global stockpile of vaccines and essential medical supplies, along with frameworks for fair allocation.
Global Surveillance and Early Warning
The agreement creates a Pandemic Surveillance Network to monitor pathogens with pandemic potential. This system will use real-time data analytics, AI-powered modeling, and decentralized information sharing across borders.
Shared Responsibilities & Accountability
Member states have committed to transparent communication, timely reporting, and participation in joint simulations and emergency drills. The agreement also includes a peer-review mechanism to evaluate national preparedness levels.
Financing Mechanism
The accord introduces a Pandemic Preparedness and Response Fund—a pooled financial resource for rapid response during outbreaks and for investing in resilient health systems, especially in low-income countries.
Technology Transfer & Intellectual Property Flexibility
To avoid the vaccine nationalism seen during COVID-19, the agreement encourages voluntary licensing, knowledge sharing, and tech transfer between countries and pharmaceutical firms, especially during declared public health emergencies.


The Road to Adoption: Challenges and Breakthroughs
The road to finalizing this historic agreement wasn’t easy. Several contentious points emerged during negotiations:
Vaccine sharing vs. national interests
Intellectual property rights vs. access
Surveillance obligations vs. data sovereignty
Despite these hurdles, WHO Director-General Dr. Tedros Adhanom Ghebreyesus praised the “spirit of solidarity” that ultimately led to consensus. Countries agreed that future pandemic security is a shared responsibility, not a national luxury.
What Happens Next?
While adoption is a significant milestone, implementation is the true test. Countries are now expected to:
Integrate the agreement into national legislation
Build or upgrade their pandemic preparedness infrastructures
Participate in WHO-led evaluations and training
Ensure inclusive public health communication strategies
The WHO will also publish regular reports to monitor compliance and support nations lagging behind in readiness.
Global Reactions: A Mixed Yet Hopeful Response
Global South Welcomes the Move
Many developing countries see this as a victory for health equity. Leaders from Africa, South Asia, and Latin America have praised the agreement for addressing vaccine injustice and promoting fair access.
Scientific Community Optimistic
Public health experts view this as a long-overdue structural change. “Pandemics don’t respect borders—now we have a document that reflects that truth,” said Dr. Maria Van Kerkhove, WHO epidemiologist.
Major Powers Remain Cautiously Onboard
Although supportive in principle, some large economies like the U.S., China, and Russia have expressed reservations on surveillance mandates and intellectual property provisions. However, all WHO members have signed the accord.
Behind the Scenes: How the Pandemic Agreement Was Negotiated
The negotiation process for the Pandemic Agreement was coordinated by the Intergovernmental Negotiating Body (INB), established in 2021 in response to COVID-19. The INB brought together public health experts, diplomats, economists, and legal scholars from all WHO member states.
Key features of the negotiation process included
Inclusive Consultations: Stakeholders such as indigenous groups, civil society organizations, and frontline healthcare workers were consulted—ensuring diverse perspectives shaped the draft.
Regional Sensitivities: The agreement reflects varying levels of healthcare infrastructure. For instance, clauses were included to provide technical assistance and technology access for low-resource nations.
Non-State Actors: NGOs, academia, and even the private sector (especially pharmaceutical companies) played advisory roles, influencing clauses on intellectual property and R&D.
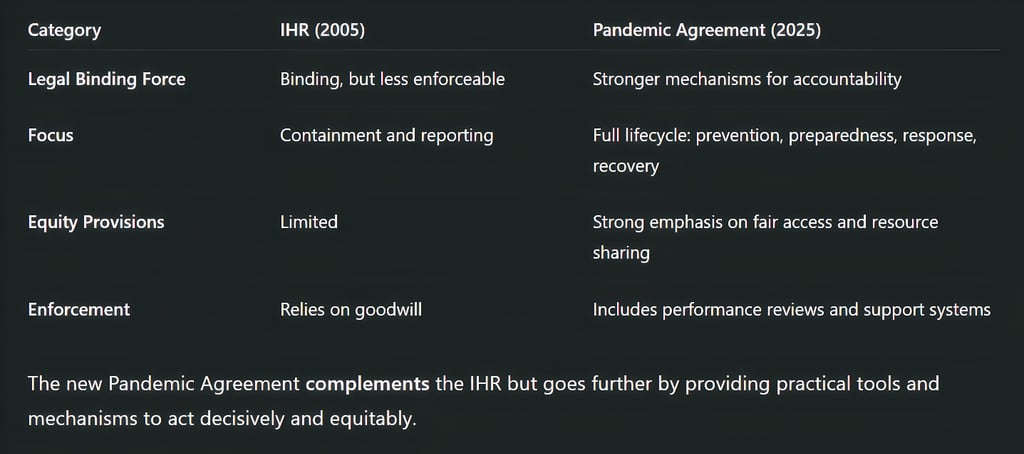
How This Agreement Differs from the International Health Regulations (IHR)
Many are wondering how this agreement differs from the International Health Regulations (2005), a legal framework already in place for global health emergencies.
Key Differences:
Digital Health Tools and AI Integration
A key innovation within the Pandemic Agreement is its integration of digital health technologies and AI-driven early warning systems.
Highlights include:
AI-powered Outbreak Prediction Models
Countries will utilize AI tools to detect anomalies in public health data that could indicate emerging threats, especially zoonotic spillovers.Digital Health Passports
A standardized, WHO-backed digital certification system for vaccination and testing during pandemics will be developed to enable safe international travel.Global Health Data Grid
A voluntary, interoperable data-sharing infrastructure to ensure real-time access to pathogen sequencing, treatment efficacy, and public health trends.
Addressing Future Unknowns: Pathogen X
The Pandemic Agreement introduces specific language on the concept of “Pathogen X”—a placeholder term for unknown future pathogens with the potential to cause pandemics.
To prepare for such threats:
Pre-pandemic R&D investments are encouraged under the new agreement, especially for platform technologies (e.g., mRNA) that can be rapidly adapted.
A "Global Scientific Task Force on Pathogen X" will be established to monitor emerging risks and advise WHO and member states.
This proactive stance recognizes that the next pandemic may not look like COVID-19—and demands flexibility in response systems.
Role of the Private Sector and Public-Private Partnerships
Unlike previous agreements, the new Pandemic Accord explicitly acknowledges the role of the private sector. From vaccine manufacturing to supply chains and digital innovation, private entities will be crucial partners.
Key mechanisms include
Pre-negotiated supply agreements with pharma companies during emergencies
Corporate accountability clauses tied to equitable distribution and data sharing
Incentives for open-source drug discovery and diagnostics
This reflects a new ethos: pandemic response is not just a public-sector effort—it’s an all-of-society mandate.


Civil Society & Public Participation
A novel feature of the agreement is its insistence on community involvement in preparedness and response. Governments are required to:
Conduct public awareness campaigns using local languages and cultural contexts
Involve grassroots health workers and community leaders in surveillance and vaccination campaigns
Provide regular public updates to maintain transparency and trust
This component aims to reduce misinformation, build local resilience, and promote behavioral compliance during future health emergencies.
Legal & Ethical Oversight
To avoid political misuse or overreach, the agreement establishes a Pandemic Ethics and Law Review Board (PELRB). This independent global body will:
Evaluate ethical concerns during outbreaks (e.g., lockdowns, surveillance, vaccine mandates)
Offer non-binding legal opinions on disputes among member states
Advocate for human rights protections even during emergency powers
This shows the WHO's commitment to balancing urgency with human dignity.
Critics & Controversies
While celebrated globally, the agreement is not without critics:
National sovereignty concerns: Some countries worry it grants too much influence to WHO over internal matters.
Vaccine manufacturers: There’s pushback from pharma companies over provisions demanding waivers or compulsory licensing.
Political polarization: In some countries, particularly in election years, the agreement is being framed as a “globalist overreach.”
Despite these challenges, the overarching global consensus remains that collaborative preparedness is preferable to chaotic individualism.
With the adoption of the world’s first Pandemic Agreement, the WHO and its member states have taken a major leap toward building a healthier, safer, and more unified world. The next step will be implementation—turning words into action, policies into practice, and pledges into protection.
Subscribe To Our Newsletter
All © Copyright reserved by Accessible-Learning Hub
| Terms & Conditions
Knowledge is power. Learn with Us. 📚


